Overview of Cannabidiolum
Scientific Name: -
Order: -
Family: -
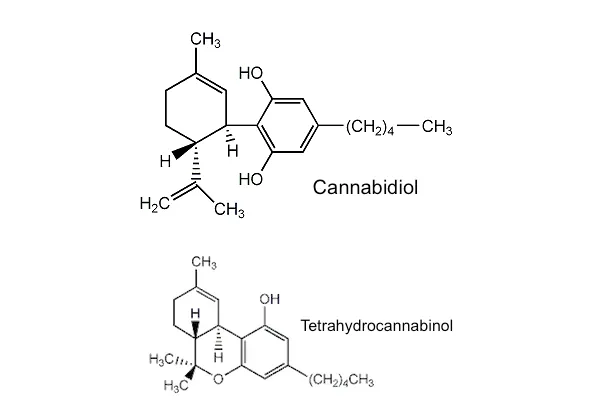
Extracts of Cannabis sativa contain cannabidiolum (also known as CBD), a cannabinoid, at concentrations of 40% or more [Bergamaschi et al. 2011]. (Cannabis indica is a synonym of Cannabis sativa.) CBD is commonly marketed as CBD oil. Unlike tetrahydrocannabinol (THC), CBD does not have psychoactive properties. CBD and THC have similar molecular weights but different structures (see compounds). In 2018, the US Food and Drug Administration (FDA) approved a highly-pure cannabidiolum extract from Cannabis for treating epilepsy. An extract containing equal amounts of CBD and THC is also approved for use in a number of European countries for treating symptoms of multiple sclerosis (MS). Most research has studied the efficacy of CBD/THC combinations in treating various health conditions. Therefore, more research is necessary to study the medicinal qualities of CBD alone. CBD is currently being marketed as a panacea (or cure-all); however, most of the claims have little to no supportive evidence, such as pain management. For the nutritional value of hemp, see hemp seeds. According to the State of California, cannabis (marijuana) smoke causes cancer and is a developmental / reproductive toxin [Proposition 65 Warning].
Strong:
CBD alone that is FDA approved:- Epilepsy in Children [2-11]
- Includes: Dravet Syndrome and Lennox-Gastaut Syndrome
Good:
CBD alone:- Counteracts the Effects of Cannabis and Tetrahydrocannabinol (THC) Intoxication and Psychosis [12-28]
Promising:
CBD alone:- Anxiety Disorders [78-80]
Conflicting (Unclear):
CBD alone:- Schizophrenia [87-90]
Limited Evidence:
CBD alone:- Cannabis Dependence and Withdrawal [91]
- Chronic Pain in Kidney Transplant Patients [92]
- Dysautonomic Syndrome after Human Papillomavirus (HPV) Vaccination [93]
- Epidermolysis Bullosa in Children, a topical CBD oil [94]
- Febrile Infection-Related Epilepsy Syndrome (FIRES) [95]
- Graft-Versus-Host Disease (GVHD) [96]
- Malignant Migrating Partial Seizures [97]
- Parkinson’s Disease [98]
- Posttraumatic Stress Disorder (PTSD) in Children [99]
- Quite Smoking (Smoking Cessation) [100]
- REM Sleep Behavior Disorder (RBD) in Patients with Parkinson’s Disease [101]
- Social Anxiety Disorder (SAD) [102-103]
- Tumors, synthetic CBD [104]
- Type 2 Diabetes, tetrahydrocannabivarin (THCV) may be more effective [105]
- ADHD [106]
- Arthritis [107]
- Cancer-Related Anorexia-Cachexia Syndrome [108]
- Central Neuropathic Pain from Brachial Plexus Avulsion [109]
- Chemotherapy-Induced Nausea and Vomiting [110]
- Chemotherapy-Induced Neuropathic Pain [111]
- Chronic Pain [112]
- Epilepsy in Children [113]
- Painful Diabetic Peripheral Neuropathy [114-115]
- Stiff-Person Syndrome (SPS) [116]
- Tourette Syndrome (TS) [117]
No Evidence:
CBD alone:- Bipolar Disorder [118]
- Chronic Pain [119]
- Crohn’s Disease [120]
- Huntington’s Disease [121]
- Glaucoma [122]
- Huntington’s Disease [123]
No Clinical Research:
- HIV
- And all other conditions.
- Decreased Appetite
- Diarrhea
- Disturbance in Attention
- Dizziness
- Drowsiness
- Dry Mouth
- Headache
- Intermittent Episodes of Feeling Hot
- Nausea
- Avoid or contact a licensed healthcare practitioner, if you have or had a history of depression, psychotic disorders, and schizophrenia. CBD may weaken the immune system and should not be taken if you have an HIV infection.
- In general, researchers have found that CBD is safer than tetrahydrocannabinol (THC) because CBD has antipsychotic-like properties and may counteract the effects of THC.
- Even though Cannabis has been associated with causing amotivational syndrome (i.e., detachment, lack of drive and emotions), taking CBD did not improve motivation.
- Since there are not enough studies on CBD and patients with schizophrenia, CBD may cause more harm than good or nothing at all.
- Schizophrenia onset may be caused by THC, but not by CBD.
- CBD has been shown to not cause chromosome damage.
- CBD does not appear to impact performance.
There is not enough research on the use of supplements containing CBD during pregnancy and breast-feeding, so consult a licensed healthcare practitioner before use or avoid use. Nonetheless, it is not recommended to take CBD during pregnancy and breast-feeding.
CBD oil supplements are not "drugs", the best doses have not been thoroughly established. Make sure to follow the specific product instructions and take as directed on the label or consult a licensed healthcare practitioner before use.
1. Bergamaschi MM, Queiroz RH, Zuardi AW, Crippa JA. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011 Sep 1;6(4):237-49. Review. PubMed PMID: 22129319. 2. Álvarez Bravo G, Yusta Izquierdo A. The adult motor phenotype of Dravet syndrome is associated with mutation of the STXBP1 gene and responds well to cannabidiol treatment. Seizure. 2018 Aug;60:68-70. doi: 10.1016/j.seizure.2018.06.010. Epub 2018 Jun 13. PubMed PMID: 29929108. 3. Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, Sanvito WL, Lander N, Mechoulam R. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21(3):175-85. PubMed PMID: 7413719. 4. Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, Miller I, Flamini R, Wilfong A, Filloux F, Wong M, Tilton N, Bruno P, Bluvstein J, Hedlund J, Kamens R, Maclean J, Nangia S, Singhal NS, Wilson CA, Patel A, Cilio MR. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016 Mar;15(3):270-8. doi: 10.1016/S1474-4422(15)00379-8. Epub 2015 Dec 24. Erratum in: Lancet Neurol. 2016 Apr;15(4):352. PubMed PMID: 26724101. 5. Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, Greenwood SM, Roberts C, Checketts D, VanLandingham KE, Zuberi SM; GWPCARE3 Study Group.. Effect of Cannabidiol on Drop Seizures in the Lennox-Gastaut Syndrome. N Engl J Med. 2018 May 17;378(20):1888-1897. doi: 10.1056/NEJMoa1714631. PubMed PMID: 29768152. 6. Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, Scheffer IE, Thiele EA, Wright S; Cannabidiol in Dravet Syndrome Study Group.. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N Engl J Med. 2017 May 25;376(21):2011-2020. doi: 10.1056/NEJMoa1611618. PubMed PMID: 28538134. 7. Hausman-Kedem M, Menascu S, Kramer U. Efficacy of CBD-enriched medical cannabis for treatment of refractory epilepsy in children and adolescents - An observational, longitudinal study. Brain Dev. 2018 Aug;40(7):544-551. doi: 10.1016/j.braindev.2018.03.013. Epub 2018 Apr 16. PubMed PMID: 29674131. 8. Sulak D, Saneto R, Goldstein B. The current status of artisanal cannabis for the treatment of epilepsy in the United States. Epilepsy Behav. 2017 May;70(Pt B):328-333. doi: 10.1016/j.yebeh.2016.12.032. Epub 2017 Feb 21. PubMed PMID: 28254350. 9. Szaflarski JP, Bebin EM, Cutter G, DeWolfe J, Dure LS, Gaston TE, Kankirawatana P, Liu Y, Singh R, Standaert DG, Thomas AE, Ver Hoef LW; UAB CBD Program.. Cannabidiol improves frequency and severity of seizures and reduces adverse events in an open-label add-on prospective study. Epilepsy Behav. 2018 Oct;87:131-136. doi: 10.1016/j.yebeh.2018.07.020. Epub 2018 Aug 9. PubMed PMID: 30100226. 10. Szaflarski M, Hansen B, Bebin EM, Szaflarski JP. Social correlates of health status, quality of life, and mood states in patients treated with cannabidiol for epilepsy. Epilepsy Behav. 2017 May;70(Pt B):364-369. doi: 10.1016/j.yebeh.2016.12.033. Epub 2017 Feb 21. PubMed PMID: 28236578. 11. Thiele EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C, Lyons PD, Taylor A, Roberts C, Sommerville K; GWPCARE4 Study Group.. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018 Mar 17;391(10125):1085-1096. doi: 10.1016/S0140-6736(18)30136-3. Epub 2018 Jan 26. PubMed PMID: 29395273. 12. Cunha Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, Nosarti C, O' Carroll CM, Seal M, Allen P, Mehta MA, Stone JM, Tunstall N, Giampietro V, Kapur S, Murray RM, Zuardi AW, Crippa JA, Atakan Z, McGuire PK. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010 Feb;35(3):764-74. doi: 10.1038/npp.2009.184. Epub 2009 Nov 18. PubMed PMID: 19924114; PubMed Central PMCID: PMC3055598. 13. Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O'Carroll C, Allen P, Seal ML, Fletcher PC, Crippa JA, Giampietro V, Mechelli A, Atakan Z, McGuire P. Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch Gen Psychiatry. 2009 Apr;66(4):442-51. doi: 10.1001/archgenpsychiatry.2009.17. PubMed PMID: 19349314. 14. Bhattacharyya S, Crippa JA, Allen P, Martin-Santos R, Borgwardt S, Fusar-Poli P, Rubia K, Kambeitz J, O'Carroll C, Seal ML, Giampietro V, Brammer M, Zuardi AW, Atakan Z, McGuire PK. Induction of psychosis by Δ9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Arch Gen Psychiatry. 2012 Jan;69(1):27-36. doi: 10.1001/archgenpsychiatry.2011.161. PubMed PMID: 22213786. 15. Bhattacharyya S, Falkenberg I, Martin-Santos R, Atakan Z, Crippa JA, Giampietro V, Brammer M, McGuire P. Cannabinoid modulation of functional connectivity within regions processing attentional salience. Neuropsychopharmacology. 2015 May;40(6):1343-52. doi: 10.1038/npp.2014.258. Epub 2014 Sep 23. PubMed PMID: 25249057; PubMed Central PMCID: PMC4397391. 16. Borgwardt SJ, Allen P, Bhattacharyya S, Fusar-Poli P, Crippa JA, Seal ML, Fraccaro V, Atakan Z, Martin-Santos R, O'Carroll C, Rubia K, McGuire PK. Neural basis of Delta-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biol Psychiatry. 2008 Dec 1;64(11):966-73. doi: 10.1016/j.biopsych.2008.05.011. Epub 2008 Jun 27. PubMed PMID: 18589404. 17. Demirakca T, Sartorius A, Ende G, Meyer N, Welzel H, Skopp G, Mann K, Hermann D. Diminished gray matter in the hippocampus of cannabis users: possible protective effects of cannabidiol. Drug Alcohol Depend. 2011 Apr 1;114(2-3):242-5. doi: 10.1016/j.drugalcdep.2010.09.020. Epub 2010 Nov 2. PubMed PMID: 21050680. 18. Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S, Stone JM, Reichenberg A, Brenneisen R, Holt D, Feilding A, Walker L, Murray RM, Kapur S. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013 Jan;27(1):19-27. doi: 10.1177/0269881112460109. Epub 2012 Oct 5. PubMed PMID: 23042808. 19. Freeman TP, Pope RA, Wall MB, Bisby JA, Luijten M, Hindocha C, Mokrysz C, Lawn W, Moss A, Bloomfield MAP, Morgan CJA, Nutt DJ, Curran HV. Cannabis Dampens the Effects of Music in Brain Regions Sensitive to Reward and Emotion. Int J Neuropsychopharmacol. 2018 Jan 1;21(1):21-32. doi: 10.1093/ijnp/pyx082. PubMed PMID: 29025134; PubMed Central PMCID: PMC5795345. 20. Fusar-Poli P, Allen P, Bhattacharyya S, Crippa JA, Mechelli A, Borgwardt S, Martin-Santos R, Seal ML, O'Carrol C, Atakan Z, Zuardi AW, McGuire P. Modulation of effective connectivity during emotional processing by Delta 9-tetrahydrocannabinol and cannabidiol. Int J Neuropsychopharmacol. 2010 May;13(4):421-32. doi: 10.1017/S1461145709990617. Epub 2009 Sep 24. PubMed PMID: 19775500. 21. Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, Seal M, Surguladze SA, O'Carrol C, Atakan Z, Zuardi AW, McGuire PK. Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry. 2009 Jan;66(1):95-105. doi: 10.1001/archgenpsychiatry.2008.519. PubMed PMID: 19124693. 22. Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, Gray KM, McRae-Clark A, Lofwall MR, Sparenborg S, Walsh SL. Oral Cannabidiol does not Alter the Subjective, Reinforcing or Cardiovascular Effects of Smoked Cannabis. Neuropsychopharmacology. 2016 Jul;41(8):1974-82. doi: 10.1038/npp.2015.367. Epub 2015 Dec 28. PubMed PMID: 26708108; PubMed Central PMCID: PMC4908634. 23. Hindocha C, Freeman TP, Schafer G, Gardener C, Das RK, Morgan CJ, Curran HV. Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: a randomised, double-blind, placebo-controlled study in cannabis users. Eur Neuropsychopharmacol. 2015 Mar;25(3):325-34. doi: 10.1016/j.euroneuro.2014.11.014. Epub 2014 Dec 5. PubMed PMID: 25534187; PubMed Central PMCID: PMC4398332. 24. Nicholson AN, Turner C, Stone BM, Robson PJ. Effect of Delta-9-tetrahydrocannabinol and cannabidiol on nocturnal sleep and early-morning behavior in young adults. J Clin Psychopharmacol. 2004 Jun;24(3):305-13. PubMed PMID: 15118485. 25. Roser P, Gallinat J, Weinberg G, Juckel G, Gorynia I, Stadelmann AM. Psychomotor performance in relation to acute oral administration of Delta9-tetrahydrocannabinol and standardized cannabis extract in healthy human subjects. Eur Arch Psychiatry Clin Neurosci. 2009 Aug;259(5):284-92. doi: 10.1007/s00406-009-0868-5. Epub 2009 Feb 17. PubMed PMID: 19224107. 26. Skosnik PD, Hajós M, Cortes-Briones JA, Edwards CR, Pittman BP, Hoffmann WE, Sewell AR, D'Souza DC, Ranganathan M. Cannabinoid receptor-mediated disruption of sensory gating and neural oscillations: A translational study in rats and humans. Neuropharmacology. 2018 Jun;135:412-423. doi: 10.1016/j.neuropharm.2018.03.036. Epub 2018 Mar 28. PubMed PMID: 29604295; PubMed Central PMCID: PMC6091633. 27. Winton-Brown TT, Allen P, Bhattacharyya S, Borgwardt SJ, Fusar-Poli P, Crippa JA, Seal ML, Martin-Santos R, Ffytche D, Zuardi AW, Atakan Z, McGuire PK. Modulation of auditory and visual processing by delta-9-tetrahydrocannabinol and cannabidiol: an FMRI study. Neuropsychopharmacology. 2011 Jun;36(7):1340-8. doi: 10.1038/npp.2011.17. Epub 2011 Mar 16. Erratum in: Neuropsychopharmacology. 2011 Jul;36(8):1778. Bhattacharrya, Sagnik [corrected to Bhattacharyya, Sagnik]. PubMed PMID: 21412224; PubMed Central PMCID: PMC3096803. 28. Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology (Berl). 1982;76(3):245-50. PubMed PMID: 6285406. 29. Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, Rivas GR, Holland RM, Muhleisen P, Norberg MM, Booth J, McGregor IS. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry. 2014 Mar;71(3):281-91. doi: 10.1001/jamapsychiatry.2013.3947. PubMed PMID: 24430917. 30. Bhardwaj AK, Allsop DJ, Copeland J, McGregor IS, Dunlop A, Shanahan M, Bruno R, Phung N, Montebello M, Sadler C, Gugusheff J, Jackson M, Luksza J, Lintzeris N; Agonist Replacement for Cannabis Dependence (ARCD) study group.. Randomised Controlled Trial (RCT) of cannabinoid replacement therapy (Nabiximols) for the management of treatment-resistant cannabis dependent patients: a study protocol. BMC Psychiatry. 2018 May 18;18(1):140. doi: 10.1186/s12888-018-1682-2. PubMed PMID: 29776349; PubMed Central PMCID: PMC5960200. 31. Morgan CJ, Freeman TP, Schafer GL, Curran HV. Cannabidiol attenuates the appetitive effects of Delta 9-tetrahydrocannabinol in humans smoking their chosen cannabis. Neuropsychopharmacology. 2010 Aug;35(9):1879-85. doi: 10.1038/npp.2010.58. Epub 2010 Apr 28. PubMed PMID: 20428110; PubMed Central PMCID: PMC2906701. 32. Trigo JM, Soliman A, Staios G, Quilty L, Fischer B, George TP, Rehm J, Selby P, Barnes AJ, Huestis MA, Le Foll B. Sativex Associated With Behavioral-Relapse Prevention Strategy as Treatment for Cannabis Dependence: A Case Series. J Addict Med. 2016 Jul-Aug;10(4):274-9. doi: 10.1097/ADM.0000000000000229. PubMed PMID: 27261670; PubMed Central PMCID: PMC5586546. 33. Trigo JM, Lagzdins D, Rehm J, Selby P, Gamaleddin I, Fischer B, Barnes AJ, Huestis MA, Le Foll B. Effects of fixed or self-titrated dosages of Sativex on cannabis withdrawal and cravings. Drug Alcohol Depend. 2016 Apr 1;161:298-306. doi: 10.1016/j.drugalcdep.2016.02.020. Epub 2016 Feb 23. PubMed PMID: 26925704; PubMed Central PMCID: PMC4878903. 34. Trigo JM, Soliman A, Quilty LC, Fischer B, Rehm J, Selby P, Barnes AJ, Huestis MA, George TP, Streiner DL, Staios G, Le Foll B. Nabiximols combined with motivational enhancement/cognitive behavioral therapy for the treatment of cannabis dependence: A pilot randomized clinical trial. PLoS One. 2018 Jan 31;13(1):e0190768. doi: 10.1371/journal.pone.0190768. eCollection 2018. PubMed PMID: 29385147; PubMed Central PMCID: PMC5791962. 35. Aragona M, Onesti E, Tomassini V, Conte A, Gupta S, Gilio F, Pantano P, Pozzilli C, Inghilleri M. Psychopathological and cognitive effects of therapeutic cannabinoids in multiple sclerosis: a double-blind, placebo controlled, crossover study. Clin Neuropharmacol. 2009 Jan-Feb;32(1):41-7. doi: 10.1097/WNF.0B013E3181633497. PubMed PMID: 18978501. 36. Carotenuto A, Iodice R, Petracca M, Inglese M, Cerillo I, Cocozza S, Saiote C, Brunetti A, Tedeschi E, Manganelli F, Orefice G. Upper motor neuron evaluation in multiple sclerosis patients treated with Sativex®. Acta Neurol Scand. 2017 Apr;135(4):442-448. doi: 10.1111/ane.12660. Epub 2016 Aug 8. PubMed PMID: 27500463. 37. Centonze D, Mori F, Koch G, Buttari F, Codecà C, Rossi S, Cencioni MT, Bari M, Fiore S, Bernardi G, Battistini L, Maccarrone M. Lack of effect of cannabis-based treatment on clinical and laboratory measures in multiple sclerosis. Neurol Sci. 2009 Dec;30(6):531-4. doi: 10.1007/s10072-009-0136-5. Epub 2009 Sep 19. PubMed PMID: 19768368. 38. Collin C, Ehler E, Waberzinek G, Alsindi Z, Davies P, Powell K, Notcutt W, O'Leary C, Ratcliffe S, Nováková I, Zapletalova O, Piková J, Ambler Z. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res. 2010 Jun;32(5):451-9. doi: 10.1179/016164109X12590518685660. Epub 2010 Mar 19. PubMed PMID: 20307378. 39. Collin C, Davies P, Mutiboko IK, Ratcliffe S; Sativex Spasticity in MS Study Group.. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol. 2007 Mar;14(3):290-6. PubMed PMID: 17355549. 40. Conte A, Bettolo CM, Onesti E, Frasca V, Iacovelli E, Gilio F, Giacomelli E, Gabriele M, Aragona M, Tomassini V, Pantano P, Pozzilli C, Inghilleri M. Cannabinoid-induced effects on the nociceptive system: a neurophysiological study in patients with secondary progressive multiple sclerosis. Eur J Pain. 2009 May;13(5):472-7. doi: 10.1016/j.ejpain.2008.05.014. Epub 2008 Jul 7. PubMed PMID: 18603457. 41. Contin M, Mancinelli L, Perrone A, Sabattini L, Mohamed S, Scandellari C, Foschi M, Vacchiano V, Lugaresi A, Riva R. Tetrahydrocannabinol/Cannabidiol Oromucosal Spray in Patients With Multiple Sclerosis: A Pilot Study on the Plasma Concentration-Effect Relationship. Clin Neuropharmacol. 2018 Sep/Oct;41(5):171-176. doi: 10.1097/WNF.0000000000000294. PubMed PMID: 30024443. 42. Deutsch SI, Rosse RB, Connor JM, Burket JA, Murphy ME, Fox FJ. Current status of cannabis treatment of multiple sclerosis with an illustrative case presentation of a patient with MS, complex vocal tics, paroxysmal dystonia, and marijuana dependence treated with dronabinol. CNS Spectr. 2008 May;13(5):393-403. PubMed PMID: 18496477. 43. Ferrè L, Nuara A, Pavan G, Radaelli M, Moiola L, Rodegher M, Colombo B, Keller Sarmiento IJ, Martinelli V, Leocani L, Martinelli Boneschi F, Comi G, Esposito F. Efficacy and safety of nabiximols (Sativex(®)) on multiple sclerosis spasticity in a real-life Italian monocentric study. Neurol Sci. 2016 Feb;37(2):235-42. doi: 10.1007/s10072-015-2392-x. Epub 2015 Oct 16. PubMed PMID: 26474875. 44. Flachenecker P, Henze T, Zettl UK. Nabiximols (THC/CBD oromucosal spray, Sativex®) in clinical practice--results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticity. Eur Neurol. 2014;71(5-6):271-9. doi: 10.1159/000357427. Epub 2014 Feb 12. PubMed PMID: 24525548. 45. Flachenecker P, Henze T, Zettl UK. Long-term effectiveness and safety of nabiximols (tetrahydrocannabinol/cannabidiol oromucosal spray) in clinical practice. Eur Neurol. 2014;72(1-2):95-102. doi: 10.1159/000360285. Epub 2014 Jun 18. PubMed PMID: 24943098. 46. Freeman RM, Adekanmi O, Waterfield MR, Waterfield AE, Wright D, Zajicek J. The effect of cannabis on urge incontinence in patients with multiple sclerosis: a multicentre, randomised placebo-controlled trial (CAMS-LUTS). Int Urogynecol J Pelvic Floor Dysfunct. 2006 Nov;17(6):636-41. Epub 2006 Mar 22. PubMed PMID: 16552618. 47. Freidel M, Tiel-Wilck K, Schreiber H, Prechtl A, Essner U, Lang M. Drug-resistant MS spasticity treatment with Sativex(®) add-on and driving ability. Acta Neurol Scand. 2015 Jan;131(1):9-16. doi: 10.1111/ane.12287. Epub 2014 Sep 11. PubMed PMID: 25208898. 48. Gras A, Broughton J. A cost-effectiveness model for the use of a cannabis-derived oromucosal spray for the treatment of spasticity in multiple sclerosis. Expert Rev Pharmacoecon Outcomes Res. 2016 Dec;16(6):771-779. Epub 2016 Feb 26. PubMed PMID: 26750641. 49. Haupts M, Vila C, Jonas A, Witte K, Álvarez-Ossorio L. Influence of Previous Failed Antispasticity Therapy on the Efficacy and Tolerability of THC:CBD Oromucosal Spray for Multiple Sclerosis Spasticity. Eur Neurol. 2016;75(5-6):236-43. doi: 10.1159/000445943. Epub 2016 May 10. PubMed PMID: 27160412. 50. Kavia RB, De Ridder D, Constantinescu CS, Stott CG, Fowler CJ. Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Mult Scler. 2010 Nov;16(11):1349-59. doi: 10.1177/1352458510378020. Epub 2010 Sep 9. PubMed PMID: 20829244. 51. Koehler J, Feneberg W, Meier M, Pöllmann W. Clinical experience with THC:CBD oromucosal spray in patients with multiple sclerosis-related spasticity. Int J Neurosci. 2014 Sep;124(9):652-6. PubMed PMID: 24392812. 52. Koehler J. Who benefits most from THC:CBD spray? Learning from clinical experience. Eur Neurol. 2014;71 Suppl 1:10-5. PubMed PMID: 24457847. 53. Langford RM, Mares J, Novotna A, Vachova M, Novakova I, Notcutt W, Ratcliffe S. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol. 2013 Apr;260(4):984-97. doi: 10.1007/s00415-012-6739-4. Epub 2012 Nov 21. PubMed PMID: 23180178. 54. Lorente Fernández L, Monte Boquet E, Pérez-Miralles F, Gil Gómez I, Escutia Roig M, Boscá Blasco I, Poveda Andrés JL, Casanova-Estruch B. Clinical experiences with cannabinoids in spasticity management in multiple sclerosis. Neurologia. 2014 Jun;29(5):257-60. doi: 10.1016/j.nrl.2013.06.014. Epub 2013 Sep 10. English, Spanish. PubMed PMID: 24035293. 55. Lus G, Cantello R, Danni MC, Rini A, Sarchielli P, Tassinari T, Signoriello E. Palatability and oral cavity tolerability of THC:CBD oromucosal spray and possible improvement measures in multiple sclerosis patients with resistant spasticity: a pilot study. Neurodegener Dis Manag. 2018 Apr;8(2):105-113. doi: 10.2217/nmt-2017-0056. Epub 2018 Apr 23. PubMed PMID: 29683408. 56. Mallada Frechín J. Effect of tetrahydrocannabinol:cannabidiol oromucosal spray on activities of daily living in multiple sclerosis patients with resistant spasticity: a retrospective, observational study. Neurodegener Dis Manag. 2018 Jun;8(3):151-159. doi: 10.2217/nmt-2017-0055. Epub 2018 May 31. PubMed PMID: 29851356. 57. Maniscalco GT, Aponte R, Bruzzese D, Guarcello G, Manzo V, Napolitano M, Moreggia O, Chiariello F, Florio C. THC/CBD oromucosal spray in patients with multiple sclerosis overactive bladder: a pilot prospective study. Neurol Sci. 2018 Jan;39(1):97-102. doi: 10.1007/s10072-017-3148-6. Epub 2017 Oct 19. PubMed PMID: 29052091. 58. Marinelli L, Mori L, Canneva S, Colombano F, Currà A, Fattapposta F, Bandini F, Capello E, Abbruzzese G, Trompetto C. The effect of cannabinoids on the stretch reflex in multiple sclerosis spasticity. Int Clin Psychopharmacol. 2016 Jul;31(4):232-9. doi: 10.1097/YIC.0000000000000126. PubMed PMID: 27003093. 59. Notcutt W, Langford R, Davies P, Ratcliffe S, Potts R. A placebo-controlled, parallel-group, randomized withdrawal study of subjects with symptoms of spasticity due to multiple sclerosis who are receiving long-term Sativex® (nabiximols). Mult Scler. 2012 Feb;18(2):219-28. doi: 10.1177/1352458511419700. Epub 2011 Aug 30. PubMed PMID: 21878454. 60. Novotna A, Mares J, Ratcliffe S, Novakova I, Vachova M, Zapletalova O, Gasperini C, Pozzilli C, Cefaro L, Comi G, Rossi P, Ambler Z, Stelmasiak Z, Erdmann A, Montalban X, Klimek A, Davies P; Sativex Spasticity Study Group.. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex(®) ), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol. 2011 Sep;18(9):1122-31. doi: 10.1111/j.1468-1331.2010.03328.x. Epub 2011 Mar 1. PubMed PMID: 21362108. 61. Paolicelli D, Direnzo V, Manni A, D'Onghia M, Tortorella C, Zoccolella S, Di Lecce V, Iaffaldano A, Trojano M. Long-Term Data of Efficacy, Safety, and Tolerability in a Real-Life Setting of THC/CBD Oromucosal Spray-Treated Multiple Sclerosis Patients. J Clin Pharmacol. 2016 Jul;56(7):845-51. doi: 10.1002/jcph.670. Epub 2015 Dec 30. PubMed PMID: 26608223. 62. Patti F. Health Authorities Data Collection of THC:CBD Oromucosal Spray (L'Agenzia Italiana del Farmaco Web Registry): Figures after 1.5 Years. Eur Neurol. 2016;75 Suppl 1:9-12. doi: 10.1159/000444236. Epub 2016 Feb 23. PubMed PMID: 26901344. 63. Patti F, Messina S, Solaro C, Amato MP, Bergamaschi R, Bonavita S, Bruno Bossio R, Brescia Morra V, Costantino GF, Cavalla P, Centonze D, Comi G, Cottone S, Danni M, Francia A, Gajofatto A, Gasperini C, Ghezzi A, Iudice A, Lus G, Maniscalco GT, Marrosu MG, Matta M, Mirabella M, Montanari E, Pozzilli C, Rovaris M, Sessa E, Spitaleri D, Trojano M, Valentino P, Zappia M; SA.FE. study group.. Efficacy and safety of cannabinoid oromucosal spray for multiple sclerosis spasticity. J Neurol Neurosurg Psychiatry. 2016 Sep;87(9):944-51. doi: 10.1136/jnnp-2015-312591. Epub 2016 May 9. PubMed PMID: 27160523; PubMed Central PMCID: PMC5013116. 64. Rog DJ, Nurmikko TJ, Young CA. Oromucosal delta9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: an uncontrolled, open-label, 2-year extension trial. Clin Ther. 2007 Sep;29(9):2068-79. PubMed PMID: 18035205. 65. Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005 Sep 27;65(6):812-9. PubMed PMID: 16186518. 66. Russo M, Naro A, Leo A, Sessa E, D'Aleo G, Bramanti P, Calabrò RS. Evaluating Sativex® in Neuropathic Pain Management: A Clinical and Neurophysiological Assessment in Multiple Sclerosis. Pain Med. 2016 Jun;17(6):1145-54. doi: 10.1093/pm/pnv080. Epub 2016 Jan 13. PubMed PMID: 26764336. 67. Serpell MG, Notcutt W, Collin C. Sativex long-term use: an open-label trial in patients with spasticity due to multiple sclerosis. J Neurol. 2013 Jan;260(1):285-95. doi: 10.1007/s00415-012-6634-z. Epub 2012 Aug 10. PubMed PMID: 22878432. 68. Slof J, Gras A. Sativex® in multiple sclerosis spasticity: a cost-effectiveness model. Expert Rev Pharmacoecon Outcomes Res. 2012 Aug;12(4):439-41. doi: 10.1586/erp.12.40. Epub 2012 Jun 8. PubMed PMID: 22681512. 69. Trojano M. THC:CBD Observational Study Data: Evolution of Resistant MS Spasticity and Associated Symptoms. Eur Neurol. 2016;75 Suppl 1:4-8. doi: 10.1159/000444235. Epub 2016 Feb 23. PubMed PMID: 26901343. 70. Trojano M, Vila C. Effectiveness and Tolerability of THC/CBD Oromucosal Spray for Multiple Sclerosis Spasticity in Italy: First Data from a Large Observational Study. Eur Neurol. 2015;74(3-4):178-85. doi: 10.1159/000441619. Epub 2015 Nov 17. PubMed PMID: 26571097. 71. Vaney C, Heinzel-Gutenbrunner M, Jobin P, Tschopp F, Gattlen B, Hagen U, Schnelle M, Reif M. Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study. Mult Scler. 2004 Aug;10(4):417-24. PubMed PMID: 15327040. 72. Vermersch P, Trojano M. Tetrahydrocannabinol:Cannabidiol Oromucosal Spray for Multiple Sclerosis-Related Resistant Spasticity in Daily Practice. Eur Neurol. 2016;76(5-6):216-226. Epub 2016 Oct 13. Erratum in: Eur Neurol. 2016;76(5-6):226. PubMed PMID: 27732980. 73. Wade DT, Makela PM, House H, Bateman C, Robson P. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult Scler. 2006 Oct;12(5):639-45. PubMed PMID: 17086911. 74. Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004 Aug;10(4):434-41. PubMed PMID: 15327042. 75. Weinkle L, Domen CH, Shelton I, Sillau S, Nair K, et al. Exploring cannabis use by patients with multiple sclerosis in a state where cannabis is legal. Mult Scler Relat Disord. 2019 Jan;27:383-390. PubMed PMID: 30502644. 76. Wright S, Duncombe P, Altman DG. Assessment of blinding to treatment allocation in studies of a cannabis-based medicine (Sativex®) in people with multiple sclerosis: a new approach. Trials. 2012 Oct 9;13:189. PubMed PMID: 23046749; PubMed Central PMCID: PMC3487910. 77. Zajicek J, Fox P, Sanders H, Wright D, Vickery J, Nunn A, Thompson A; UK MS Research Group.. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo-controlled trial. Lancet. 2003 Nov 8;362(9395):1517-26. PubMed PMID: 14615106. 78. Crippa JA, Zuardi AW, Garrido GE, Wichert-Ana L, Guarnieri R, Ferrari L, Azevedo-Marques PM, Hallak JE, McGuire PK, Filho Busatto G. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology. 2004 Feb;29(2):417-26. PubMed PMID: 14583744. 79. Das RK, Kamboj SK, Ramadas M, Yogan K, Gupta V, Redman E, Curran HV, Morgan CJ. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology (Berl). 2013 Apr;226(4):781-92. doi: 10.1007/s00213-012-2955-y. Epub 2013 Jan 10. PubMed PMID: 23307069. 80. Zuardi AW, Cosme RA, Graeff FG, Guimarães FS. Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol. 1993 Jan;7(1 Suppl):82-8. doi: 10.1177/026988119300700112. PubMed PMID: 22290374. 81. Hoggart B, Ratcliffe S, Ehler E, Simpson KH, Hovorka J, et al. A multicentre, open-label, follow-on study to assess the long-term maintenance of effect, tolerance and safety of THC/CBD oromucosal spray in the management of neuropathic pain. J Neurol. 2015 Jan;262(1):27-40. PubMed PMID: 25270679. 82. Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, et al. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain. 2007 Dec 15;133(1-3):210-20. PubMed PMID: 17997224. 83. Serpell M, Ratcliffe S, Hovorka J, Schofield M, Taylor L, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain. 2014 Aug;18(7):999-1012. PubMed PMID: 24420962. 84. Johnson JR, Lossignol D, Burnell-Nugent M, Fallon MT. An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manage. 2013 Aug;46(2):207-18. PubMed PMID: 23141881. 85. Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, et al. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage. 2010 Feb;39(2):167-79. PubMed PMID: 19896326. 86. Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012 May;13(5):438-49. PubMed PMID: 22483680. 87. Boggs DL, Surti T, Gupta A, Gupta S, Niciu M, Pittman B, Schnakenberg Martin AM, Thurnauer H, Davies A, D'Souza DC, Ranganathan M. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018 Jul;235(7):1923-1932. doi: 10.1007/s00213-018-4885-9. Epub 2018 Apr 5. PubMed PMID: 29619533. 88. Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, Klosterkötter J, Hellmich M, Koethe D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012 Mar 20;2:e94. doi: 10.1038/tp.2012.15. PubMed PMID: 22832859; PubMed Central PMCID: PMC3316151. 89. McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, Taylor A, Wright S. Cannabidiol (CBD) as an Adjunctive Therapy in Schizophrenia: A Multicenter Randomized Controlled Trial. Am J Psychiatry. 2018 Mar 1;175(3):225-231. doi: 10.1176/appi.ajp.2017.17030325. Epub 2017 Dec 15. PubMed PMID: 29241357. 90. Zuardi AW, Hallak JE, Dursun SM, Morais SL, Sanches RF, Musty RE, Crippa JA. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006 Sep;20(5):683-6. Epub 2006 Jan 9. PubMed PMID: 16401651. 91. Crippa JA, Hallak JE, Machado-de-Sousa JP, Queiroz RH, Bergamaschi M, et al. Cannabidiol for the treatment of cannabis withdrawal syndrome: a case report. J Clin Pharm Ther. 2013 Apr;38(2):162-4. PubMed PMID: 23095052. 92. Cuñetti L, Manzo L, Peyraube R, Arnaiz J, Curi L, Orihuela S. Chronic Pain Treatment With Cannabidiol in Kidney Transplant Patients in Uruguay. Transplant Proc. 2018 Mar;50(2):461-464. doi: 10.1016/j.transproceed.2017.12.042. PubMed PMID: 29579828. 93. Palmieri B, Laurino C, Vadalà M. Short-Term Efficacy of CBD-Enriched Hemp Oil in Girls with Dysautonomic Syndrome after Human Papillomavirus Vaccination. Isr Med Assoc J. 2017 Feb;19(2):79-84. PubMed PMID: 28457055. 94. Chelliah MP, Zinn Z, Khuu P, Teng JMC. Self-initiated use of topical cannabidiol oil for epidermolysis bullosa. Pediatr Dermatol. 2018 Jul;35(4):e224-e227. doi: 10.1111/pde.13545. Epub 2018 May 22. PubMed PMID: 29786144. 95. Gofshteyn JS, Wilfong A, Devinsky O, Bluvstein J, Charuta J, Ciliberto MA, Laux L, Marsh ED. Cannabidiol as a Potential Treatment for Febrile Infection-Related Epilepsy Syndrome (FIRES) in the Acute and Chronic Phases. J Child Neurol. 2017 Jan;32(1):35-40. doi: 10.1177/0883073816669450. Epub 2016 Sep 29. PubMed PMID: 27655472. 96. Yeshurun M, Shpilberg O, Herscovici C, Shargian L, Dreyer J, Peck A, Israeli M, Levy-Assaraf M, Gruenewald T, Mechoulam R, Raanani P, Ram R. Cannabidiol for the Prevention of Graft-versus-Host-Disease after Allogeneic Hematopoietic Cell Transplantation: Results of a Phase II Study. Biol Blood Marrow Transplant. 2015 Oct;21(10):1770-5. doi: 10.1016/j.bbmt.2015.05.018. Epub 2015 May 30. PubMed PMID: 26033282. 97. Saade D, Joshi C. Pure cannabidiol in the treatment of malignant migrating partial seizures in infancy: a case report. Pediatr Neurol. 2015 May;52(5):544-7. doi: 10.1016/j.pediatrneurol.2015.02.008. Epub 2015 Feb 19. PubMed PMID: 25882081. 98. Chagas MH, Zuardi AW, Tumas V, Pena-Pereira MA, Sobreira ET, Bergamaschi MM, dos Santos AC, Teixeira AL, Hallak JE, Crippa JA. Effects of cannabidiol in the treatment of patients with Parkinson's disease: an exploratory double-blind trial. J Psychopharmacol. 2014 Nov;28(11):1088-98. doi: 10.1177/0269881114550355. Epub 2014 Sep 18. PubMed PMID: 25237116. 99. Shannon S, Opila-Lehman J. Effectiveness of Cannabidiol Oil for Pediatric Anxiety and Insomnia as Part of Posttraumatic Stress Disorder: A Case Report. Perm J. 2016 Fall;20(4):16-005. doi: 10.7812/TPP/16-005. Epub 2016 Oct 12. PubMed PMID: 27768570; PubMed Central PMCID: PMC5101100. 100. Morgan CJ, Das RK, Joye A, Curran HV, Kamboj SK. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict Behav. 2013 Sep;38(9):2433-6. doi: 10.1016/j.addbeh.2013.03.011. Epub 2013 Apr 1. PubMed PMID: 23685330. 101. Chagas MH, Eckeli AL, Zuardi AW, Pena-Pereira MA, Sobreira-Neto MA, Sobreira ET, Camilo MR, Bergamaschi MM, Schenck CH, Hallak JE, Tumas V, Crippa JA. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson's disease patients: a case series. J Clin Pharm Ther. 2014 Oct;39(5):564-6. doi: 10.1111/jcpt.12179. Epub 2014 May 21. PubMed PMID: 24845114. 102. Bergamaschi MM, Queiroz RH, Chagas MH, de Oliveira DC, De Martinis BS, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology. 2011 May;36(6):1219-26. PubMed PMID: 21307846; PubMed Central PMCID: PMC3079847. 103. Crippa JA, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FL, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011 Jan;25(1):121-30. PubMed PMID: 20829306. 104. Kenyon J, Liu W, Dalgleish A. Report of Objective Clinical Responses of Cancer Patients to Pharmaceutical-grade Synthetic Cannabidiol. Anticancer Res. 2018 Oct;38(10):5831-5835. doi: 10.21873/anticanres.12924. PubMed PMID: 30275207. 105. Jadoon KA, Ratcliffe SH, Barrett DA, Thomas EL, Stott C, Bell JD, O'Sullivan SE, Tan GD. Efficacy and Safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and Lipid Parameters in Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Pilot Study. Diabetes Care. 2016 Oct;39(10):1777-86. doi: 10.2337/dc16-0650. Epub 2016 Aug 29. PubMed PMID: 27573936. 106. Cooper RE, Williams E, Seegobin S, Tye C, Kuntsi J, Asherson P. Cannabinoids in attention-deficit/hyperactivity disorder: A randomised-controlled trial. Eur Neuropsychopharmacol. 2017 Aug;27(8):795-808. doi: 10.1016/j.euroneuro.2017.05.005. Epub 2017 May 30. PubMed PMID: 28576350. 107. Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford). 2006 Jan;45(1):50-2. Epub 2005 Nov 9. PubMed PMID: 16282192. 108. Cannabis-In-Cachexia-Study-Group., Strasser F, Luftner D, Possinger K, Ernst G, Ruhstaller T, Meissner W, Ko YD, Schnelle M, Reif M, Cerny T. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol. 2006 Jul 20;24(21):3394-400. PubMed PMID: 16849753. 109. Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain. 2004 Dec;112(3):299-306. PubMed PMID: 15561385. 110. Duran M, Pérez E, Abanades S, Vidal X, Saura C, Majem M, Arriola E, Rabanal M, Pastor A, Farré M, Rams N, Laporte JR, Capellà D. Preliminary efficacy and safety of an oromucosal standardized cannabis extract in chemotherapy-induced nausea and vomiting. Br J Clin Pharmacol. 2010 Nov;70(5):656-63. doi: 10.1111/j.1365-2125.2010.03743.x. PubMed PMID: 21039759; PubMed Central PMCID: PMC2997305. 111. Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. 2014 Jan;47(1):166-73. doi: 10.1016/j.jpainsymman.2013.02.018. Epub 2013 Jun 4. PubMed PMID: 23742737. 112. Wade DT, Robson P, House H, Makela P, Aram J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil. 2003 Feb;17(1):21-9. PubMed PMID: 12617376. 113. Maa E, Figi P. The case for medical marijuana in epilepsy. Epilepsia. 2014 Jun;55(6):783-6. doi: 10.1111/epi.12610. Epub 2014 May 22. PubMed PMID: 24854149. 114. Hoggart B, Ratcliffe S, Ehler E, Simpson KH, Hovorka J, et al. A multicentre, open-label, follow-on study to assess the long-term maintenance of effect, tolerance and safety of THC/CBD oromucosal spray in the management of neuropathic pain. J Neurol. 2015 Jan;262(1):27-40. PubMed PMID: 25270679. 115. Selvarajah D, Gandhi R, Emery CJ, Tesfaye S. Randomized placebo-controlled double-blind clinical trial of cannabis-based medicinal product (Sativex) in painful diabetic neuropathy: depression is a major confounding factor. Diabetes Care. 2010 Jan;33(1):128-30. PubMed PMID: 19808912; PubMed Central PMCID: PMC2797957. 116. Vicente-Valor MI, Garcia-Llopis P, Mejia Andujar L, Antonino de la Camara G, García del Busto N, Lopez Tinoco MJ, Quintana Vergara B, Peiro Vilaplana C, Dominguez Moran JA, Sánchez Alcaraz A. Cannabis derivatives therapy for a seronegative stiff-person syndrome: a case report. J Clin Pharm Ther. 2013 Feb;38(1):71-3. doi: 10.1111/j.1365-2710.2012.01365.x. Epub 2012 Jun 21. PubMed PMID: 22726074. 117. Jakubovski E, Müller-Vahl K. Speechlessness in Gilles de la Tourette Syndrome: Cannabis-Based Medicines Improve Severe Vocal Blocking Tics in Two Patients. Int J Mol Sci. 2017 Aug 10;18(8). pii: E1739. doi: 10.3390/ijms18081739. PubMed PMID: 28796166; PubMed Central PMCID: PMC5578129. 118. Zuardi A, Crippa J, Dursun S, Morais S, Vilela J, Sanches R, Hallak J. Cannabidiol was ineffective for manic episode of bipolar affective disorder. J Psychopharmacol. 2010 Jan;24(1):135-7. doi: 10.1177/0269881108096521. Epub 2008 Sep 18. PubMed PMID: 18801823. 119. Notcutt W, Price M, Miller R, Newport S, Phillips C, Simmons S, Sansom C. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 'N of 1' studies. Anaesthesia. 2004 May;59(5):440-52. PubMed PMID: 15096238. 120. Naftali T, Mechulam R, Marii A, Gabay G, Stein A, Bronshtain M, Laish I, Benjaminov F, Konikoff FM. Low-Dose Cannabidiol Is Safe but Not Effective in the Treatment for Crohn's Disease, a Randomized Controlled Trial. Dig Dis Sci. 2017 Jun;62(6):1615-1620. doi: 10.1007/s10620-017-4540-z. Epub 2017 Mar 27. PubMed PMID: 28349233. 121. Consroe P, Laguna J, Allender J, Snider S, Stern L, Sandyk R, Kennedy K, Schram K. Controlled clinical trial of cannabidiol in Huntington's disease. Pharmacol Biochem Behav. 1991 Nov;40(3):701-8. PubMed PMID: 1839644. 122. Tomida I, Azuara-Blanco A, House H, Flint M, Pertwee RG, Robson PJ. Effect of sublingual application of cannabinoids on intraocular pressure: a pilot study. J Glaucoma. 2006 Oct;15(5):349-53. PubMed PMID: 16988594. 123. López-Sendón Moreno JL, García Caldentey J, Trigo Cubillo P, Ruiz Romero C, García Ribas G, Alonso Arias MA, García de Yébenes MJ, Tolón RM, Galve-Roperh I, Sagredo O, Valdeolivas S, Resel E, Ortega-Gutierrez S, García-Bermejo ML, Fernández Ruiz J, Guzmán M, García de Yébenes Prous J. A double-blind, randomized, cross-over, placebo-controlled, pilot trial with Sativex in Huntington's disease. J Neurol. 2016 Jul;263(7):1390-400. doi: 10.1007/s00415-016-8145-9. Epub 2016 May 9. PubMed PMID: 27159993. 124. Belgrave BE, Bird KD, Chesher GB, Jackson DM, Lubbe KE, Starmer GA, Teo RK. The effect of cannabidiol, alone and in combination with ethanol, on human performance. Psychopharmacology (Berl). 1979 Aug 8;64(2):243-6. PubMed PMID: 115049. 125. Bergamaschi MM, Queiroz RH, Zuardi AW, Crippa JA. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011 Sep 1;6(4):237-49. Review. PubMed PMID: 22129319. 126. Cuñetti L, Manzo L, Peyraube R, Arnaiz J, Curi L, Orihuela S. Chronic Pain Treatment With Cannabidiol in Kidney Transplant Patients in Uruguay. Transplant Proc. 2018 Mar;50(2):461-464. doi: 10.1016/j.transproceed.2017.12.042. PubMed PMID: 29579828. 127. Dalton WS, Martz R, Lemberger L, Rodda BE, Forney RB. Influence of cannabidiol on delta-9-tetrahydrocannabinol effects. Clin Pharmacol Ther. 1976 Mar;19(3):300-9. PubMed PMID: 770048. 128. Guinguis R, Ruiz MI, Rada G. Is cannabidiol an effective treatment for schizophrenia? Medwave. 2017 Aug 9;17(7):e7010. doi: 10.5867/medwave.2017.07.7010. Spanish, English. PubMed PMID: 28820868. 129. Ilan AB, Gevins A, Coleman M, ElSohly MA, de Wit H. Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behav Pharmacol. 2005 Sep;16(5-6):487-96. PubMed PMID: 16148455. 130. Juckel G, Roser P, Nadulski T, Stadelmann AM, Gallinat J. Acute effects of Delta9-tetrahydrocannabinol and standardized cannabis extract on the auditory evoked mismatch negativity. Schizophr Res. 2007 Dec;97(1-3):109-17. Epub 2007 Sep 19. PubMed PMID: 17884351. 131. Kafil TS, Nguyen TM, MacDonald JK, Chande N. Cannabis for the treatment of ulcerative colitis. Cochrane Database Syst Rev. 2018 Nov 8;11:CD012954. doi: 10.1002/14651858.CD012954.pub2. PubMed PMID: 30406638. 132. Lawn W, Freeman TP, Pope RA, Joye A, Harvey L, Hindocha C, Mokrysz C, Moss A, Wall MB, Bloomfield MA, Das RK, Morgan CJ, Nutt DJ, Curran HV. Acute and chronic effects of cannabinoids on effort-related decision-making and reward learning: an evaluation of the cannabis 'amotivational' hypotheses. Psychopharmacology (Berl). 2016 Oct;233(19-20):3537-52. doi: 10.1007/s00213-016-4383-x. Epub 2016 Sep 2. PubMed PMID: 27585792; PubMed Central PMCID: PMC5021728. 133. Martin-Santos R, Crippa JA, Batalla A, Bhattacharyya S, Atakan Z, Borgwardt S, Allen P, Seal M, Langohr K, Farré M, Zuardi AW, McGuire PK. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr Pharm Des. 2012;18(32):4966-79. Review. PubMed PMID: 22716148. 134. Matsuyama SS, Fu TK. In vivo cytogenetic effects of cannabinoids. J Clin Psychopharmacol. 1981 May;1(3):135-40. PubMed PMID: 6271848. 135. Morgan CJA, Freeman TP, Hindocha C, Schafer G, Gardner C, Curran HV. Individual and combined effects of acute delta-9-tetrahydrocannabinol and cannabidiol on psychotomimetic symptoms and memory function. Transl Psychiatry. 2018 Sep 5;8(1):181. doi: 10.1038/s41398-018-0191-x. PubMed PMID: 30185793; PubMed Central PMCID: PMC6125482. 136. Roser P, Haussleiter IS. Antipsychotic-like effects of cannabidiol and rimonabant: systematic review of animal and human studies. Curr Pharm Des. 2012;18(32):5141-55. Review. PubMed PMID: 22716153. 137. Selvarajah D, Gandhi R, Emery CJ, Tesfaye S. Randomized placebo-controlled double-blind clinical trial of cannabis-based medicinal product (Sativex) in painful diabetic neuropathy: depression is a major confounding factor. Diabetes Care. 2010 Jan;33(1):128-30. doi: 10.2337/dc09-1029. Epub 2009 Oct 6. PubMed PMID: 19808912; PubMed Central PMCID: PMC2797957. 138. van Amsterdam J, Vervloet J, de Weert G, Buwalda VJA, Goudriaan AE, van den Brink W. Acceptance of pharmaceutical cannabis substitution by cannabis using patients with schizophrenia. Harm Reduct J. 2018 Sep 20;15(1):47. doi: 10.1186/s12954-018-0253-7. PubMed PMID: 30236118; PubMed Central PMCID: PMC6149068. 139. Bergamaschi MM, Queiroz RH, Zuardi AW, Crippa JA. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011 Sep 1;6(4):237-49. Review. PubMed PMID: 22129319. 140. Consroe P, Carlini EA, Zwicker AP, Lacerda LA. Interaction of cannabidiol and alcohol in humans. Psychopharmacology (Berl). 1979;66(1):45-50. PubMed PMID: 120541. 141. Dalton WS, Martz R, Lemberger L, Rodda BE, Forney RB. Influence of cannabidiol on delta-9-tetrahydrocannabinol effects. Clin Pharmacol Ther. 1976 Mar;19(3):300-9. PubMed PMID: 770048. 142. Dalton WS, Martz R, Rodda BE, Lemberger L, Forney RB. Influence of cannabidiol on secobarbital effects and plasma kinetics. Clin Pharmacol Ther. 1976 Dec;20(6):695-700. PubMed PMID: 791563. 143. Hallak JE, Dursun SM, Bosi DC, de Macedo LR, Machado-de-Sousa JP, Abrão J, Crippa JA, McGuire P, Krystal JH, Baker GB, Zuardi AW. The interplay of cannabinoid and NMDA glutamate receptor systems in humans: preliminary evidence of interactive effects of cannabidiol and ketamine in healthy human subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2011 Jan 15;35(1):198-202. doi: 10.1016/j.pnpbp.2010.11.002. Epub 2010 Nov 7. PubMed PMID: 21062637. 144. Holland ML, Allen JD, Arnold JC. Interaction of plant cannabinoids with the multidrug transporter ABCC1 (MRP1). Eur J Pharmacol. 2008 Sep 4;591(1-3):128-31. doi: 10.1016/j.ejphar.2008.06.079. Epub 2008 Jun 27. PubMed PMID: 18619955. 145. Manini AF, Yiannoulos G, Bergamaschi MM, Hernandez S, Olmedo R, Barnes AJ, Winkel G, Sinha R, Jutras-Aswad D, Huestis MA, Hurd YL. Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans. J Addict Med. 2015 May-Jun;9(3):204-10. doi: 10.1097/ADM.0000000000000118. PubMed PMID: 25748562; PubMed Central PMCID: PMC4449284. 146. Yamaori S, Koeda K, Kushihara M, Hada Y, Yamamoto I, Watanabe K. Comparison in the in vitro inhibitory effects of major phytocannabinoids and polycyclic aromatic hydrocarbons contained in marijuana smoke on cytochrome P450 2C9 activity. Drug Metab Pharmacokinet. 2012;27(3):294-300. Epub 2011 Dec 13. PubMed PMID: 22166891. 147. Yamaori S, Ebisawa J, Okushima Y, Yamamoto I, Watanabe K. Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: role of phenolic hydroxyl groups in the resorcinol moiety. Life Sci. 2011 Apr 11;88(15-16):730-6. doi: 10.1016/j.lfs.2011.02.017. Epub 2011 Feb 26. PubMed PMID: 21356216. 148. Afendi FM, Okada T, Yamazaki M, Hirai-Morita A, Nakamura Y, Nakamura K, Ikeda S, Takahashi H, Altaf-Ul-Amin M, Darusman LK, Saito K, Kanaya S. KNApSAcK family databases: integrated metabolite-plant species databases for multifaceted plant research. Plant Cell Physiol. 2012 Feb;53(2):e1.